In a groundbreaking medical development, Janusz Racz, a 67-year-old lung cancer patient, has become the first person to receive a new lung cancer vaccine as part of a global clinical trial. The trial, taking place across multiple countries, is testing the effectiveness of the mRNA-based vaccine developed by BioNTech, a German biotechnology firm.
About the Vaccine: BNT116
The experimental vaccine, known as BNT116, is designed to treat non-small cell lung cancer (NSCLC), the most common type of lung cancer. It uses messenger RNA (mRNA) technology to instruct the immune system to recognize and attack cancer cells. Unlike traditional treatments like chemotherapy, which can damage both cancerous and healthy cells, BNT116 specifically targets tumor markers on cancer cells, minimizing harm to healthy tissue.
| Key Vaccine Details | Description |
|---|---|
| Developer | BioNTech |
| Vaccine Name | BNT116 |
| Target | Non-small cell lung cancer (NSCLC) |
| Technology | mRNA-based immunotherapy |
| Goal | Activate the immune system to attack cancer cells while sparing healthy tissue |
How the Vaccine Works
The mRNA technology in BNT116 exposes the patient’s immune system to specific tumor markers associated with NSCLC. By recognizing these markers, the immune system learns to identify and combat cancer cells carrying them. Over several weeks, patients receive multiple injections, each with a unique RNA sequence aimed at boosting the immune response to cancer cells.
| How BNT116 Works | Description |
|---|---|
| Immune Activation | mRNA instructs immune cells to recognize NSCLC-associated tumor markers |
| Treatment Regimen | Weekly injections for 6 weeks, followed by every 3 weeks for 54 weeks |
| Specific Targeting | Focuses on cancer cells to minimize harm to healthy cells |
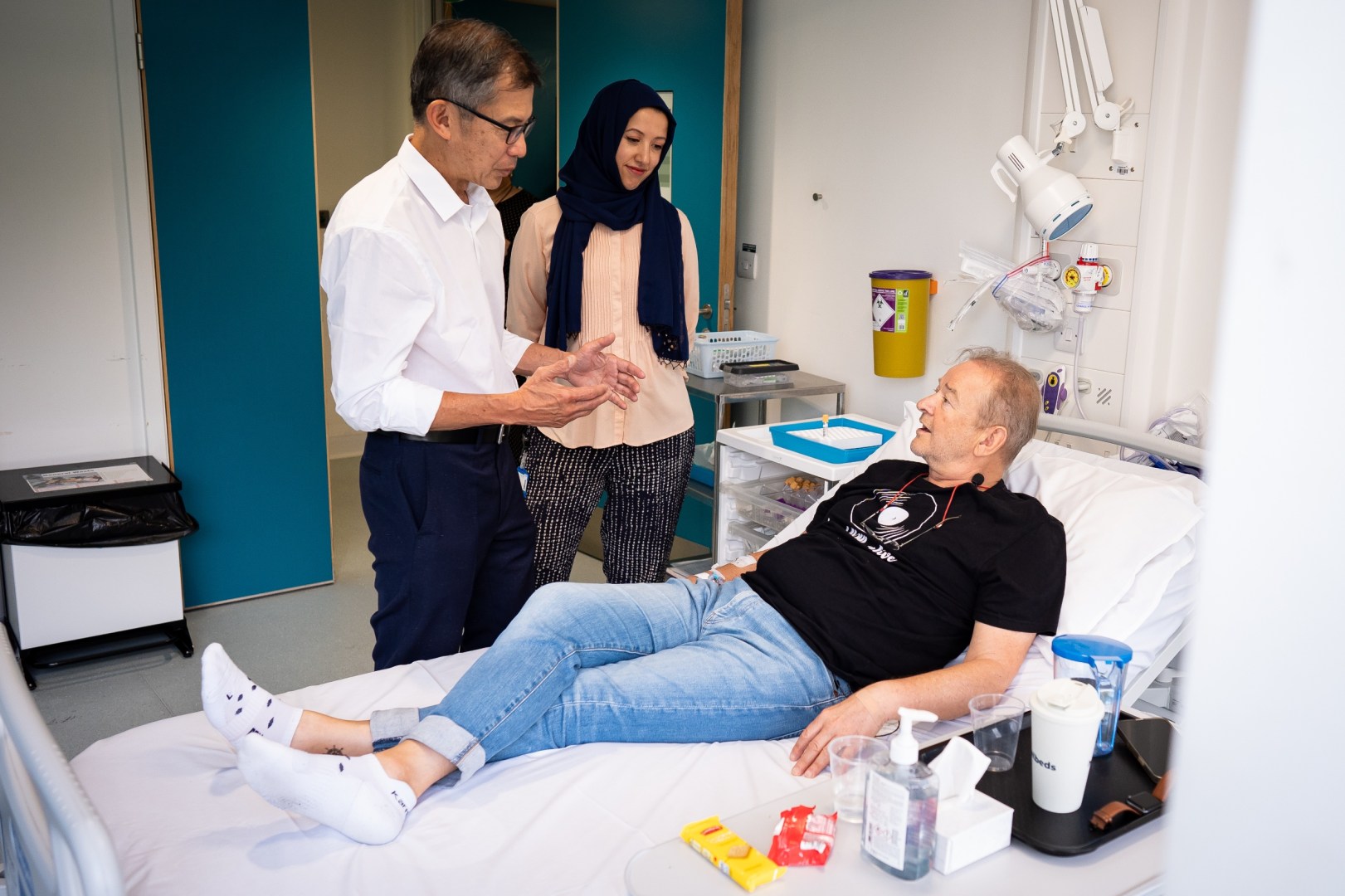
First Participant: Janusz Racz
Janusz Racz, the first patient to receive the vaccine, was diagnosed with lung cancer in May 2024. His treatment started with chemotherapy and radiation therapy. On Tuesday, Racz received the first of six consecutive injections at the National Institute for Health Research UCLH Clinical Research Facility. Each injection was administered five minutes apart, with the entire process taking just 30 minutes.
Racz is optimistic about his participation in the trial, stating:
"As a scientist myself, I know that science can only advance if people agree to participate in programs like this... My family did research about the trial too, and they supported me taking part."
| Janusz Racz | Details |
|---|---|
| Age | 67 years old |
| Diagnosis | Lung cancer (May 2024) |
| Trial Participation | First participant in the BNT116 lung cancer vaccine trial |
| Treatment Timeline | First dose in August 2024; ongoing weekly doses for 6 weeks, then every 3 weeks |
Trial and Research Team
The clinical trial, led by Siow Ming Lee, a consultant medical oncologist at University College London Hospitals (UCLH), marks the beginning of a new era in mRNA-based immunotherapy. The study aims to establish the safety and tolerability of the BNT116 vaccine and will enroll approximately 130 patients with NSCLC across 34 research sites in seven countries, including the UK, US, Germany, Hungary, Poland, Spain, and Turkey.
| Key Trial Details | Description |
|---|---|
| Number of Participants | Approximately 130 |
| Research Sites | 34 sites in 7 countries |
| Trial Duration | 54 weeks |
| Lead Researcher | Siow Ming Lee, UCLH |
Importance of the Trial
Lung cancer remains the leading cause of cancer deaths globally, with an estimated 1.8 million deaths in 2020 alone. The BNT116 vaccine trial represents a significant advancement in the search for immunotherapies that target cancer cells more precisely, reducing the side effects commonly associated with traditional cancer treatments.
| Global Impact of Lung Cancer | Details |
|---|---|
| Leading Cause of Death | Lung cancer is responsible for 1.8 million deaths worldwide (2020) |
| Current Treatments | Chemotherapy, radiation therapy; high risk of damage to healthy cells |
| Vaccine Goal | Develop a treatment that targets cancer cells while sparing healthy tissue |
Looking Ahead
As the first lung cancer vaccine to enter human trials, BNT116 could represent a new frontier in cancer treatment. This trial will assess its potential to extend the health span of patients with NSCLC while minimizing the damaging effects of traditional cancer therapies. With 130 patients participating across multiple countries, the trial is expected to yield important data that could transform the landscape of lung cancer treatment.
More Information:
- Title: 67-Year-Old Receives World’s First Lung Cancer Vaccine in Human Trials
- Published By: Mrigakshi Dixit
- Source: UCLH Clinical Research Facility Press Release
- Acknowledgements: The Guardian, Interesting Engineering
💛 Support the Editor
If you liked this post and would like to support the editor through a financial donation then you can do so below!
💛 Donate












Discussion