A groundbreaking study by Professor Thai-Yen Ling at National Taiwan University has presented a novel method for addressing acute liver failure through the engineering of small extracellular vesicles (sEVs) using click chemistry. Published in the Journal of Extracellular Vesicles, this research highlights the innovative approach in targeting liver cells with precision, thus enhancing the effectiveness of therapeutic interventions.
Understanding the Context of Liver Failure
The liver plays a vital role in metabolizing the nutrients we consume and processing medications. However, the excessive intake of common analgesics, particularly acetaminophen (paracetamol), can lead to severe hepatic dysfunction. In critical situations, this can result in acute liver failure (ALF), a rapidly advancing, life-threatening condition that often requires a liver transplant for successful recovery.
Limitations of Current Treatments
The conventional treatment for acetaminophen overdose involves the administration of N-acetylcysteine (NAC). Although NAC can mitigate hepatic injury, its effectiveness is not guaranteed in severe cases, and it can introduce potential risks, such as:
- Allergic reactions
- Interference with liver regeneration when used over extended periods
- Overall insufficient action in critical instances
The Novel Approach: Engineering sEVs
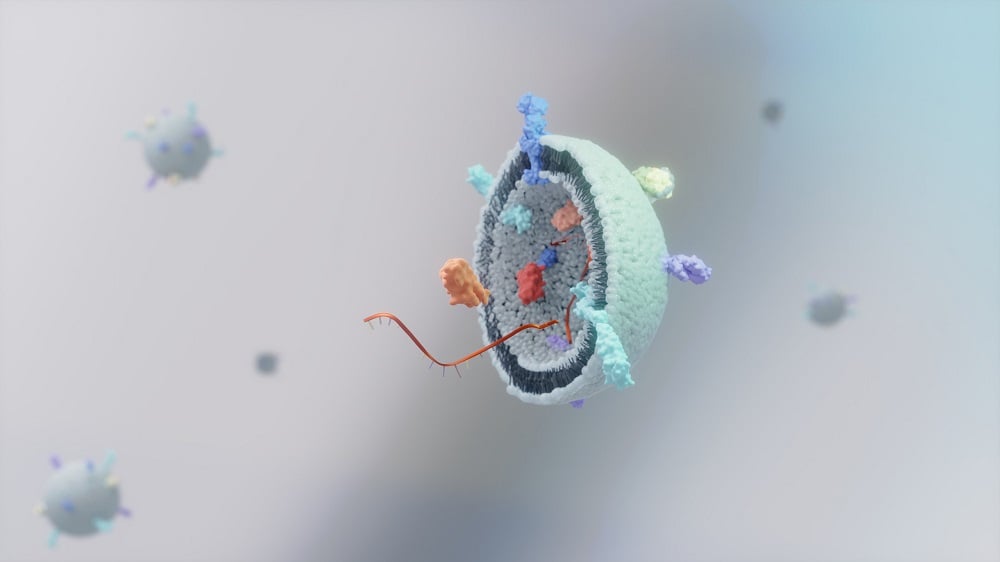
To overcome the limitations associated with NAC, Professor Ling's team aimed to explore small extracellular vesicles (sEVs). These minute natural particles are essential for intercellular communication, facilitating the transport of critical biomolecules such as RNA, proteins, and lipids, which are integral to cellular healing processes.
Utilizing Click Chemistry for Enhanced Targeting
A significant challenge in using sEVs therapeutically has been their lack of targeting, causing them to disperse and accumulate in non-target organs. To tackle this issue, the researchers employed click chemistry, a selective and efficient method for joining molecules without disrupting biological systems. This process is particularly effective for modifying biological particles, such as sEVs.
The development process involved several key steps:
- Labeling of vesicles: The team tagged vesicles produced by placenta-derived mesenchymal stromal cells (pcMSCs) using a sugar-based molecule known as Ac4ManNAz.
- Antibody fragment engineering: A small antibody fragment, modified to bind specifically to the ASGR1 protein found on liver cells, was conjugated using click chemistry.
- Formation of CAR-sEVs: The resultant vesicles, now termed CAR-sEVs, were equipped with therapeutic payloads and displayed enhanced targeting capability for hepatic tissue.
Experimental Validation and Efficacy
In preclinical trials, **CAR-sEVs** exhibited promising outcomes:
| Parameter | CAR-sEVs | Unmodified sEVs |
|---|---|---|
| Targeting Efficiency | Significantly increased | Standard |
| Hepatic Inflammation Reduction | Marked reduction | Minimal effect |
| Tissue Repair Promotion | Substantial | Insufficient |
These findings indicate that the engineered vesicles not only home in on damaged liver areas more effectively but also promote healing, making them a compelling alternative to traditional cell-based therapies.
Advantages of CAR-sEVs Over Traditional Treatments
One significant advantage of using CAR-sEVs is the **cell-free** nature of this therapy, which eliminates the risks commonly associated with whole-cell transplants, including:
- Immune rejection
- Uncontrolled cell proliferation
Future Applications and Versatility
Beyond addressing acute liver failure, the versatility of this click chemistry-based approach offers potential for targeting other tissues or organs. This raises the possibility of developing therapies aimed at various conditions, including:
- Cardiovascular diseases
- Cancer
- Neurological disorders
“This approach demonstrates how precise chemical engineering can transform natural cellular messages into targeted therapies.” – Prof. Thai-Yen Ling
Conclusion
In conclusion, the study conducted by Professor Thai-Yen Ling's team signifies a remarkable advancement in the treatment of acute liver failure through the innovative use of CAR-sEVs. By leveraging the capabilities of click chemistry, this research paves the way for developing tailored therapies that enhance efficacy, reduce side effects, and hold significant promise for a multitude of medical applications.
Literature Cited
Yen‐Ting Lu et al, Small Extracellular Vesicles Engineered Using Click Chemistry to Express Chimeric Antigen Receptors Show Enhanced Efficacy in Acute Liver Failure, Journal of Extracellular Vesicles (2025).
For more information, visit Science X.











Discussion